Real patients, real results
If you're thinking, “What could improved walking mean to me?” it may help to see people with MS-related walking difficulty who had improvement in their walking with AMPYRA. AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg, is the first and only brand prescription medicine indicated to help improve walking in adults with multiple sclerosis (MS). This was demonstrated by an increase in walking speed.
AMPYRA does not work for everyone, and people experience different levels of response to the medication.
Who should not take AMPYRA® (dalfampridine)?
Do not take AMPYRA if you have ever had a seizure, have certain types of kidney problems, or are allergic to dalfampridine (4-aminopyridine), the active ingredient in AMPYRA.
The Timed 25-Foot Walk and AMPYRA
In clinical trials, the effectiveness of AMPYRA was assessed using the Timed 25-Foot Walk (T25FW), a simple and reliable measure of walking ability and a standard assessment used to evaluate walking in people with MS.
During a T25FW, patients are asked to walk 25 feet as quickly and safely as possible while timed in their doctor's office. The resulting score helps doctors evaluate walking speed.
The response shown in these videos is typical of a person who responded to AMPYRA in the clinical trials. Please note that this is a depiction of improvement in walking at a single point in time.
Some patients experienced improvement in their walking ability within a couple of weeks. Others noticed improvement up to 6 weeks after starting.
AMPYRA does not work for everyone, and people experience different levels of response to the medication.
According to a 2011 study, it takes approximately 2.8 to 5.2 seconds for an average person without MS to complete the T25FW. If you think your walking may not be up to speed, ask your doctor if AMPYRA may be right for you.
Timed 25-Foot Walk: Before and After AMPYRA® (dalfampridine)
The people shown in these videos performed a Timed 25-foot Walk during 2 doctor's office visits: before taking AMPYRA and again after having been on treatment. The T25FW is a simple and reliable measure of walking ability and a standard assessment doctors use to evaluate walking speed.
Selected Important Safety Information
Before taking AMPYRA, tell your doctor if you are taking any other prescription or OTC medicines, such as cimetidine.
View videos of actual patients
-

MS Type: Relapsing remitting Age: 60 years Gender: Female MS Disease Duration: Not reported Occupation: Volunteer Baseline Walking Time: 10.74 seconds Baseline Walking Speed: 2.33 feet per second Walking Time After 9 Weeks on Treatment: 8.28 seconds Walking Speed After 9 Weeks on Treatment: 3.02 feet per second Average Increase in Speed: 29.61% 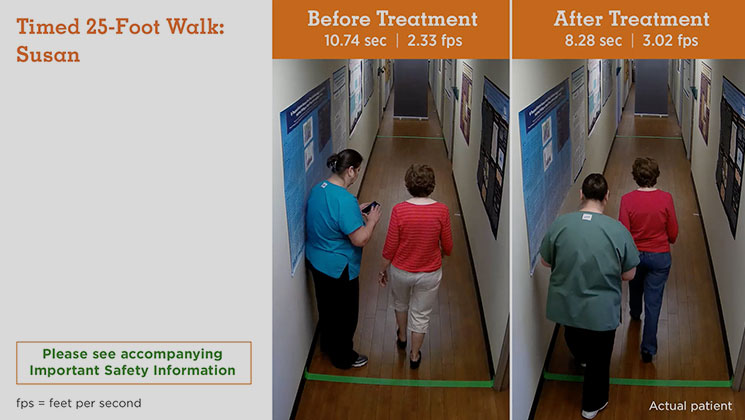
-

MS Type: Secondary progressive Age: 58 years Gender: Male MS Disease Duration: 11 years Occupation: Lumber Sales Baseline Walking Time: 10.22 seconds Baseline Walking Speed: 2.45 feet per second Walking Time After 6 Weeks on Treatment: 7.86 seconds Walking Speed After 6 Weeks on Treatment: 3.18 feet per second Average Increase in Speed: 29.80% 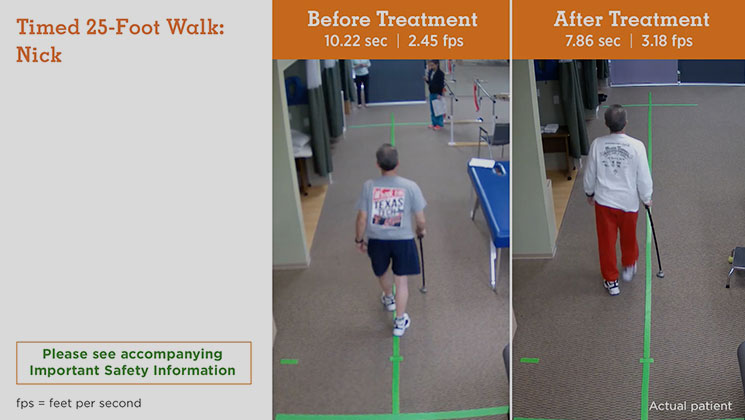
-

MS Type: Relapsing remitting Age: 42 years Gender: Female MS Disease Duration: 14 years Occupation: N/A Baseline Walking Time: 11.26 seconds Baseline Walking Speed: 2.22 feet per second Walking Time After 12 Weeks on Treatment: 8.80 seconds Walking Speed After 12 Weeks on Treatment: 2.84 feet per second Average Increase in Speed: 27.93% 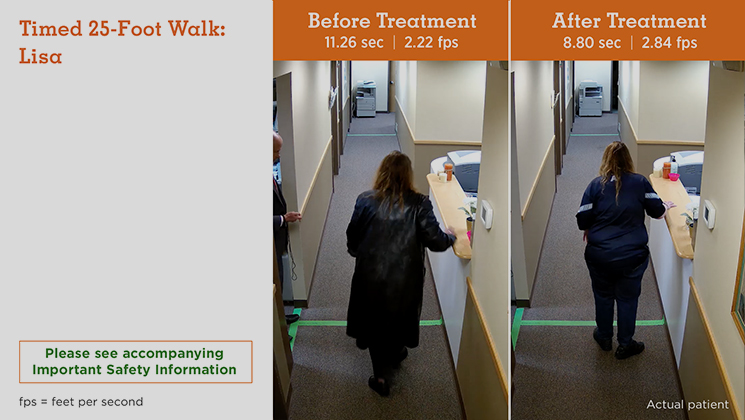
Indication
AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg, is the first and only brand prescription medicine indicated to help improve walking in adults with multiple sclerosis (MS). This was demonstrated by an increase in walking speed.
Important Safety Information
Do not take AMPYRA if you have ever had a seizure, have certain types of kidney problems, or are allergic to dalfampridine (4-aminopyridine), the active ingredient in AMPYRA.
Indication & Important Safety Information
INDICATION
AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg, is the first and only brand prescription medicine indicated to help improve walking in adults with multiple sclerosis (MS). This was demonstrated by an increase in walking speed.
IMPORTANT SAFETY INFORMATION
Do not take AMPYRA if you:
- have ever had a seizure,
- have certain types of kidney problems, or
- are allergic to dalfampridine (4-aminopyridine), the active ingredient in AMPYRA.
Take AMPYRA exactly as prescribed by your doctor.
Before taking AMPYRA, tell your doctor if you:
- have any other medical conditions
- are taking compounded 4-aminopyridine
- are taking any other prescription or OTC medicines, such as cimetidine
- are pregnant or plan to become pregnant. It is not known if AMPYRA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if AMPYRA passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you take AMPYRA.
Stop taking AMPYRA and call your doctor right away if you have a seizure while taking AMPYRA. You could have a seizure even if you never had a seizure before. Your chance of having a seizure is higher if you take too much AMPYRA or if your kidneys have a mild decrease of function, which is common after age 50. Your doctor may do a blood test to check how well your kidneys are working before you start AMPYRA.
AMPYRA should not be taken with other forms of 4-aminopyridine (4-AP, fampridine), since the active ingredient is the same.
AMPYRA may cause dizziness or vertigo. If you have these symptoms do not drive, operate machinery or do other dangerous activities.
AMPYRA may cause serious side effects, including severe allergic reactions. Stop taking AMPYRA and call your doctor right away or get emergency medical help if you have shortness of breath or trouble breathing, swelling of your throat or tongue, or hives.
The most common side effects for AMPYRA in MS patients were urinary tract infection; trouble sleeping; dizziness; headache; nausea; weakness; back pain; problems with balance; multiple sclerosis relapse; burning, tingling, or itching of your skin; irritation in your nose and throat; constipation; indigestion; and pain in your throat.
Please see the Patient Medication Guide.







